Alkaline batteries have literally become an essential part of our lives. They are used in everything from hearing aids to remote controls, toys and video games. In fact, the industry has grown into a multi-billion dollar industry since its inception in the 1950s. The market for alkaline batteries is expected to grow to a value of 8 billion euros by the year 2022.
However, it was not always this way. One of the first batteries to make its way into commercial use was the lead acetate battery. It was invented in 1859, but still remains the primary technology in rechargeable car batteries. The alkaline battery as we know it today was first introduced in 1959. It is this battery, with some refinements to its technology over time, that has become widely popular due to its cost and effectiveness.
How alkaline batteries work
Alkaline batteries are basically a contained chemical reaction. Each cell consists of an anode, a cathode and an electrolyte material that facilitates a transfer of electrons. In a standard alkaline battery, the cathode is made of manganese dioxide, which forms a layer on the inside of the battery casing. The anode consists of zinc powder dispersed in an electrolyte solution. Potassium hydroxide is the standard electrolyte solution for alkaline batteries. Other components in a battery include the separator to keep the anode and cathode electrically separated and a current collector to capture electrons.
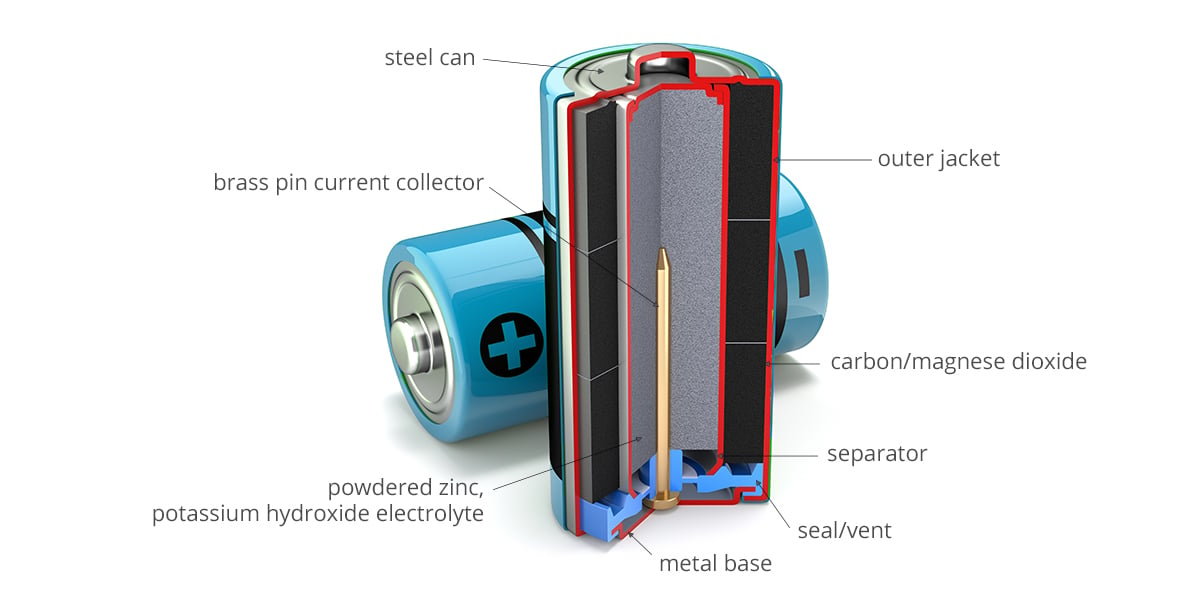
When an alkaline battery is wired into a circuit, the chemical reaction takes place. A reduction reaction takes place at the cathode to produce hydroxyl ions. At the same time, the zinc anode is oxidised by consuming hydroxyl ions during which electrons are released. It is these electrons that power electrical devices.
An alkaline battery goes flat when the resources to complete the chemical reaction are depleted. The first chemical to run out is the magnesium dioxide, which means that no more hydroxyl ions can be formed, and no more electrons released.
Factors affecting the performance of alkaline batteries
It is important to note that the voltage of a battery will reduce over its life. This is acceptable because electrical devices can function normally in a range from 0.9 to 1.5 volts.
Battery manufacturers describe the operation of alkaline batteries and the factors that affect their performance as follows:
- The colder the temperature, the less efficient an alkaline battery is. Cold temperatures inhibit the movement of ions. The slow-down in chemical activity reduces the battery voltage while the current drawn remains constant.
- Another factor affecting the life of an alkaline battery is the amount of current drawn. The greater the load on a battery, the quicker the chemicals will be depleted, and the battery will go flat.
Advantages of potassium hydroxide alkaline batteries
Prior to the invention of alkaline batteries, the most common type of battery in use was the zinc carbon battery. But, the performance of alkaline batteries far exceeds the earlier technology. They have double the energy intensity and last between four to nine times longer. Alkaline batteries also have a very good shelf life – lasting up to ten years without experiencing a noticeable deterioration in performance.
Rechargeable batteries like nickel-metal hydrogen batteries and lithium ion batteries have grown in popularity due to the reduction in waste from recharging. Lithium ion batteries are high performance batteries, which are able to deliver a significantly higher capacity than alkaline batteries. They are also much more expensive, which makes them less suitable for many of the day to day electronic uses of alkaline batteries.
Alkaline batteries waste and recycling
Potassium hydroxide alkaline batteries are not harmful to the environment. They do not contain toxic chemicals like mercury, which are controlled substances. As such, they can be disposed of as non-hazardous waste. However, it is always better to recycle materials than send them to landfills where they will slowly decompose. Each alkaline battery contains small amounts of zinc, manganese and steel, each of which can be reused if recovered.
Contact Vynova
Vynova is a leading European producer of potassium hydroxide. We have manufacturing sites in Belgium and France. Our production processes use the best available technology ensuring efficient performance and environmental responsibility. Contact one of our potassium sales representatives here or view our website to find out more about us.




